Explain the Differences Between Strong and Weak Acids and Bases
Rves of Strong and Weak Acids and Bases. Weak acids vs strong acids GCSE Chemistry only Strong and weak acids.
Difference Between Strong And Weak Acids Definition Properties Examples
The main difference between strong acidsbases with weak acidsbases is that strong acid will have most of their molecules ionized.

. Strong acids are good conductors of electricity on contrary weak acids are not too good as conductors of electricity. They get completely ionized split up into ions in. Strong acids and strong bases react completely to produce salt and water.
H aq OH-aq H 2 O l. One of the other differences between strong and weak acids and bases is in measurements like the enthalpy of neutralization. The aqueous solution is one of the examples that include a base and its conjugate acid.
That means strong acid will produce more ions compared to the same amount of weak acid. Main Differences Between Strong Acid and Weak Acid. Lesson 1 Acids and Bases At the end of the lesson you should be able to.
Titration Curves of Pre-lab questions. Remember that neutralization is the reaction. Bases have pH values from 7 to 14.
Strong acid add all their H to will weak acid only add some H to solution. Difference between Strong and Weak Base. Strong bases are chemical compounds that completely dissociate in aqueous solutions forming OH ions.
An acid is a molecule or other species which can donate a proton or accept an electron pair in reactions. Strong acids have at least 2 or more oxygens than hydrogens. Strong and weak acidsbases.
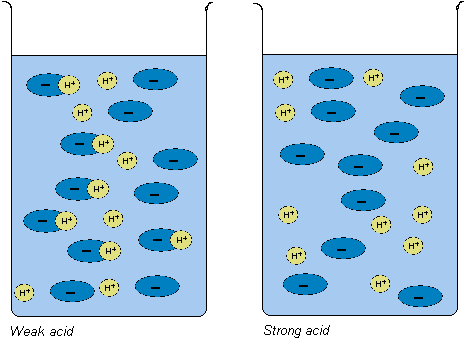
Strong acid passes electricity faster whereas weak acids are slow conductors. It will pass less current than weak acid. Weak acids and weak bases do not react completely as they are not completely dissociating.
Relies on the concentration of the hydronium ions. This trait also helps strong acid to conduct electricity as they have more ions. It contains less unpair atoms hence its conductivity is lower.
Weak acid possesses lower conductivity due to presence of less unpair atoms. All strong acidsbases have identical strength as far as the degree of dissociation goes because they all completely dissociate to H 3 O or OH-solutions. Explain the different definitions of acids and bases and discus the limitations of each 2.
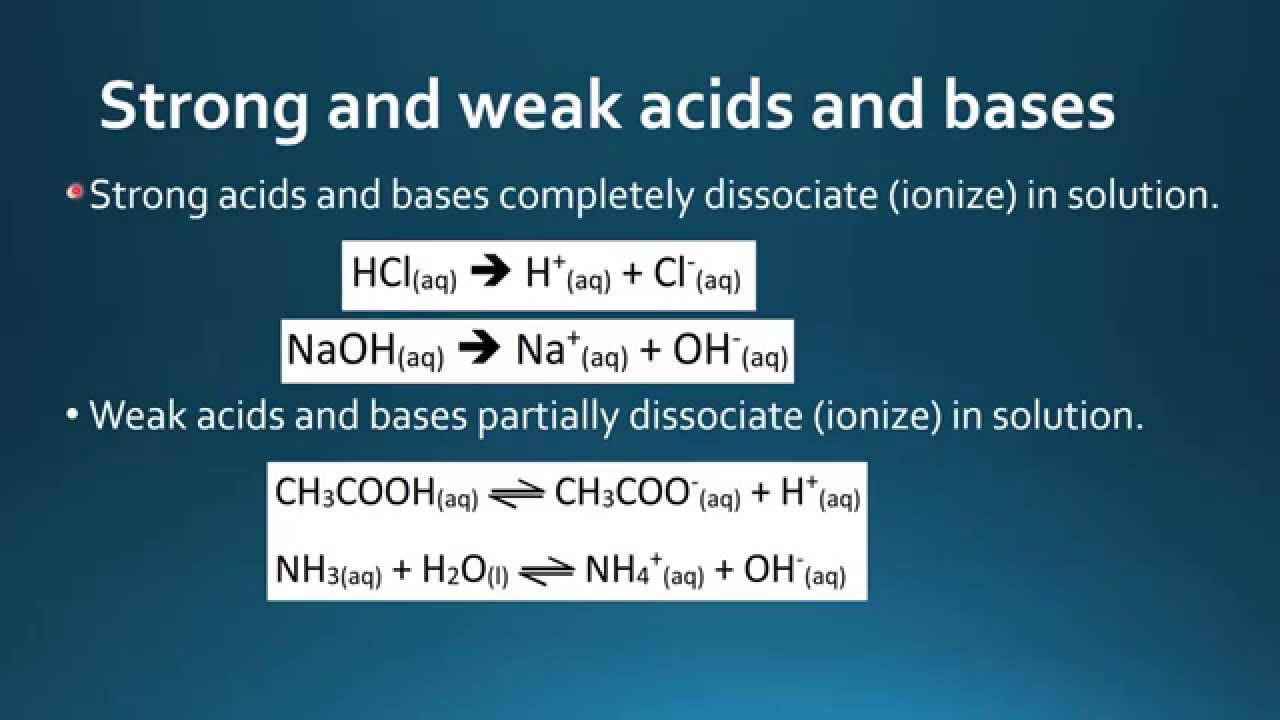
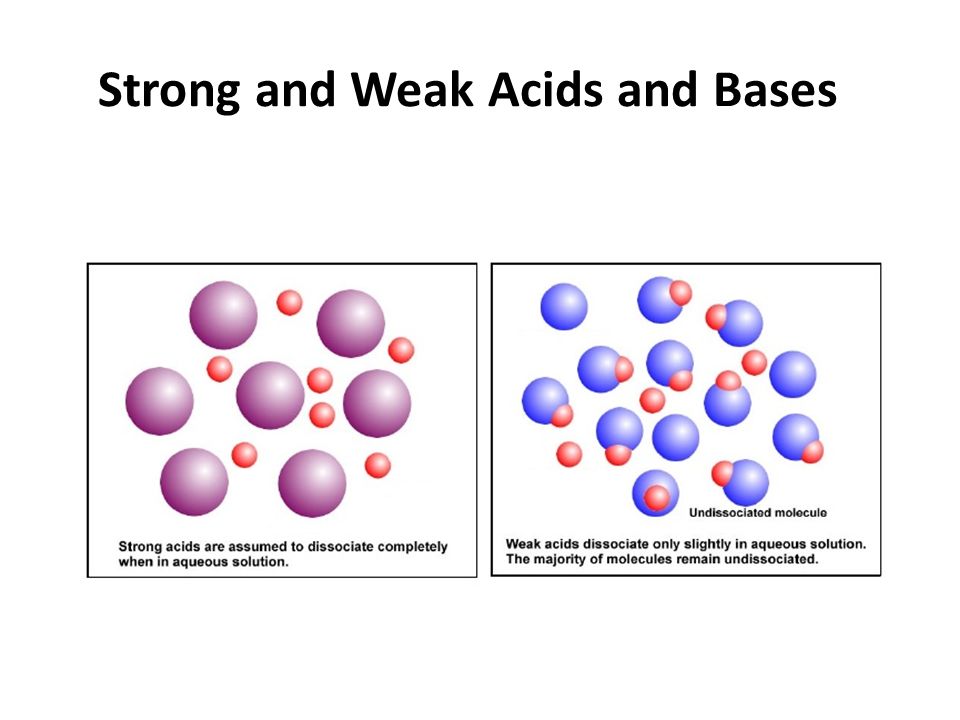
Strong acids are acids that completely dissociate in aqueous solutions releasing H ions. Created by Ram Prakash. Strong acids dissociate fully in water to produce the maximum number of.
The acidity merely determines the speed of reaction an acid has with other chemicals but the chemical properties of strong and weak acids are pretty much the same. The main difference between a Strong base and a Weak base is that a strong base ionized completely or 100 in an acid-base reaction or water or solution whereas a weak base is incapable of completely ionizing in a reaction or a solution it may ionize 1 or 99. Those bases which do not fully ionise in the aqueous solution are considered to be weak bases.
Acids are classified into two groups known as strong acids and weak acids. Rate of reaction is faster for strong acids. An acid is any chemical compound once dissolved in water produces a solution with hydrogen ion activity more than purified water.
Calculate the pH of an HC Solution in which the H01 -175 x 10M. It is still said to be a weak base. Strong acid is an acid that ionize completely while weak acid partially ionize.
Stronger acids are close to 1 and weak acids are close to 7. There are two major types of acids as strong acids and weak acids. The strong bases are closer to 14 and weak bases are closer to 7.
Operational Definition of Acids and Bases Acids and bases can be defined. Discuss the concept of conjugate pairs and 3. Because the rate of reaction depends upon the degree of dissociation alpha and strong acids have higher degrees of dissociation.
A weak acid only partially dissociates from its salt. Weak Base These bases completely dissociate in water. Weak acidsbases can produce ions when mixed into water.
Acids have pH values from 1 to 7. The main difference between strong and weak acids is that strong acids dissociate completely in aqueous solutions whereas weak acids. Examples of strong bases are sodium hydroxide NaOH and potassium hydroxide KOH.
Bases which fully ionise and liberate hydroxide ions OH- when dissolved in water are called strong bases. The main difference between strong acidsbases with weak acidsbases is that strong acid will have most of their molecules ionized. Strong and weak acids It is important to understand and differentiate between the terms concentration and strength when referring to acids or bases Acidity is caused by the presence of hydrogen ions in the solution.
Based on the concentration of the acid molecules in the aqueous solution these acids can be in two. Strong acids and strong bases react completely to produce salt and water. The pH will rise normally at first but as it reaches a zone where the solution seems to be buffered the slope levels out.
After this zone the pH rises sharply through its equivalence point and levels out again like the strong acidstrong base reaction. Main Difference Strong vs Weak Acids. Strong acidsbases dissociate completely whereas weak acidsbases dissociate partially.
Explain the difference between a stre xplain the difference between a strong acid and a weak acid. Differentiate strong and weak acids and bases and their relation to acid-base equilibria 71. The main difference between strong and weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are partially dissociated to give some of the hydroxyl ions it has.
Strong base Besides the special acids like HCl or HF how can you tell the difference between a strong or weak acid. Stronger acids are close to 1 and weak acids are close to 7. That means strong acid will produce more ions compared to the same amount of weak acid.
Calculate the pH of a NAOH solution in which the OH 37 x 10 M. A base is an aqueous substance that could absorb hydrogen ions. Relies on the concentration of the hydroxide ions.
Strong Base It completely dissociates into its ions in water or in a compound that can remove a proton H from a weak acid. Answer 1 of 15. Strong acids react faster whereas weak acids take time to react with any base.
What Is The Brief Difference Between Titration Of Strong Acid To Strong Base And Weak Acid To A Strong Base Quora

Difference Between Strong And Weak Base With Examples In Table

Strong And Weak Acids Bases Video Khan Academy
8 3 3 Distinguish Between Strong And Weak Acids And Bases Youtube

Strong Weak Acids And Bases Youtube

Strong And Weak Acids And Bases Ppt Video Online Download

Titration Curves Of Strong And Weak Acids And Bases Pre Lab Questions Sed And Weak Acids Ng Acids Cont 214 2343 Homeworklib

8 4 Distinguish Between Strong And Weak Acids And Bases Sl Youtube

Weak Acid Weak Base Reactions Video Khan Academy
Acids And Bases For High School
Strong And Weak Acids And Bases Ppt Video Online Download

8 3 3 Distinguish Between Strong And Weak Acids And Bases Youtube

Strong Weak Acids Bases Mr Carson S Science Page

Strong Acids And Bases Mcat Chemistry Cheat Sheet Study Guide Studypk Chemistry Education Chemistry Lessons Teaching Chemistry
Difference Between Weak Base And Strong Base Difference Between

List Of Strong Acids More Than 8 Examples With Images Teachoo

Difference Between Weak And Strong Acid Compare The Difference Between Similar Terms


Comments
Post a Comment